Background: Relapsed/refractory (R/R) DLBCL constitutes 40% of all DLBCL cases. Coventional salvage regimens, such as R-ICE, R-GDP, and R-DHAP, have an overall response rate (ORR) of 60% and a complete response rate (CR) of 30-40%. For patients who have achieved complete metabolic remission as determined by PET-CT evaluation after salvage therapy, undergoing autologous transplantation is crucial in order to achieve durable long-term survival. The outcomes of these R/R DLBCL remain to be improved.
Aims: This prospective study aims to investigate whether adding novel agents to R-ICE (R-ICE-X) based on genetic subtypes could improve clinical efficacy in R/R DLBCL. This trial is registered at clinicaltrials.gov (NCT05348213).
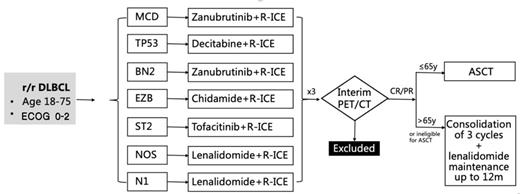
Method:In this study, patients with R/R DLBCL aged 18 -75 years were enrolled. All patients were assigned and stratified by genetic subtype and received different targeted agents combined with R-ICE. The efficacy was evaluated after 3 courses of R-ICE-X (every 21 days). Patients with CR/PR were subsequently treated with autologous hematopoietic stem cell transplantation or 3 courses of R-ICE-X consolidation and lenalidomide maintenance for up to 12 months. See the detail in the figure. The primary endpoint was the overall response rate (ORR), and the secondary endpoints were the 2-year progression-free survival (PFS) rate, 2-year overall survival (OS) rate, and safety evaluation.
Results: At the time of data cut-off, a total of 66 patients were enrolled, with a median age of 61(20-75) years. In the patients with R-ICE-zanubrutinib group (n=28), the ORR was 84.0%, with 15 patients (15/25, 60.0%) achieved CR and 6 patients (6/25, 24.0%) achieved PR. The 1-year PFS rate and OS rate were 68.2% and 90.5%, respectively. In the patients with lenalidomide combined with R-ICE (n=28), 16 patients (16/26, 61.5%) achieved CR, 4 patients (4/26, 15.4%) achieved PR, and the ORR was 76.9%. The 1-year PFS and OS rates were 78.5% and 100.0%, respectively. In the patients with the R-ICE-decitabine group (n=7), the ORR was 66.7%, with 4 patients (4/6, 66.7%) achieving CR. The 1-year PFS rate and OS rate were 50.0% and 60.0%, respectively. The number of other groups needs to be further updated. Among 66 patients, 17 patients had autologous transplantation (ASCT) and 1-year PFS and OS of patients with ASCT were both 87.1%. The number of other groups will be further updated.
Conclusion: Novel targeted agents in combination with R-ICE (R-ICE-X) regimen was well tolerated and efficacy in R/R DLBCL patients, meanwhile, it could be a promising bridging regimen for ASCT. The study is ongoing and further results will be continuously released.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal